Amorphous materials, such as plastic and glass, have always intrigued scientists due to their unique properties. Unlike crystalline solids, these materials do not form orderly structures when cooled. Instead, they exist in a state that resembles a supercooled liquid, flowing extremely slowly. The microscopic mechanism behind the rigidity of these materials has remained a mystery for a long time. However, researchers at the Department of Energy’s Lawrence Berkeley National Laboratory have recently made a groundbreaking discovery that sheds light on this hidden phase transition.
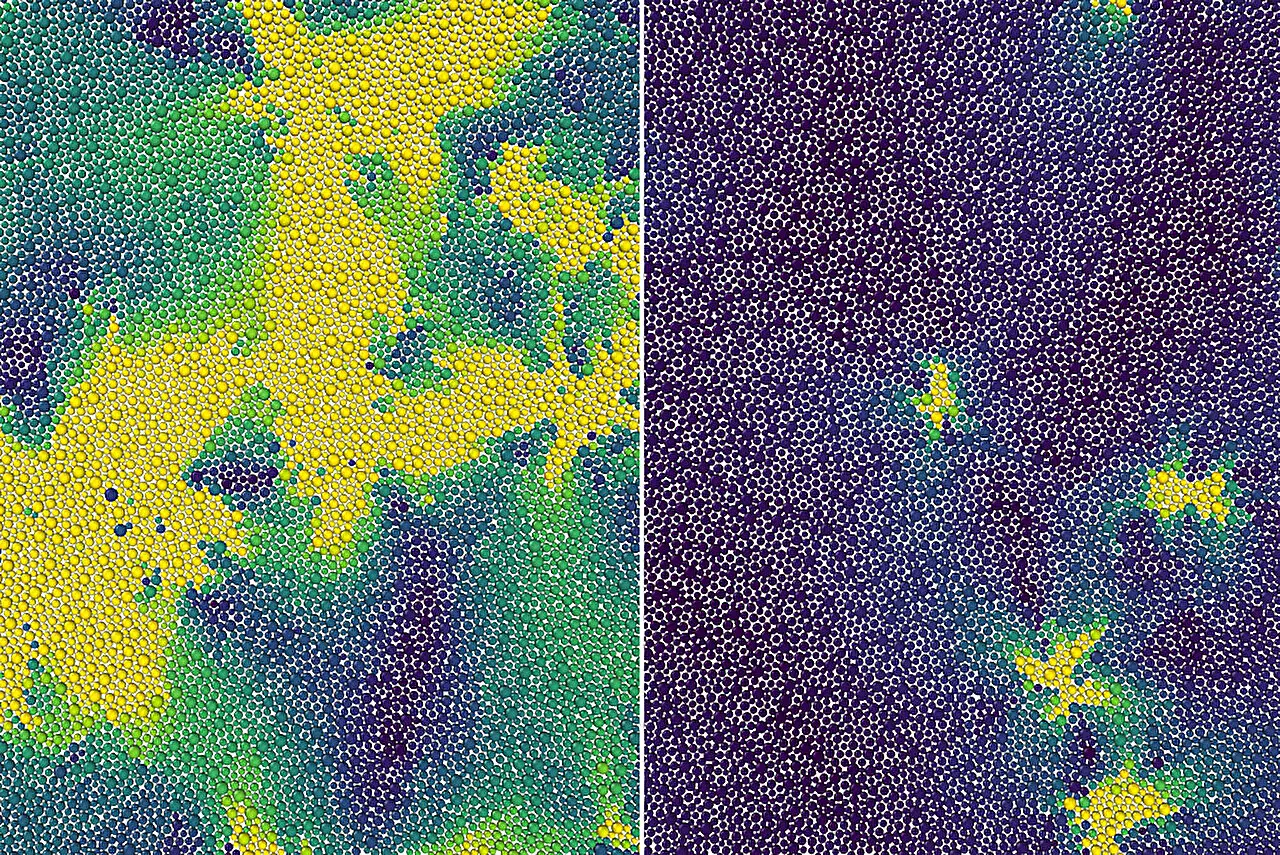
Through a combination of theory, computer simulations, and previous experiments, the scientists have uncovered the reason why amorphous materials become rigid at the microscopic level. They explain that as these materials are cooled, the molecules remain in a disordered state, resembling a liquid, until they reach a specific temperature called the onset temperature. At this critical point, the molecules become extremely viscous and barely move, marking the onset of rigidity. This novel phase transition, previously unknown, distinguishes supercooled liquids from normal liquids.
One of the key concepts in their theory is the idea of excitations. Supercooled liquids constantly transition between various configurations of molecules, resulting in localized particle movements. To explain this phenomenon, the researchers treated the excitations in a 2D supercooled liquid as defects in a crystalline solid. They propose that as the temperature increases towards the onset temperature, every bound pair of defects breaks apart into an unbounded pair. This unbinding process is what causes the system to lose its rigidity and exhibit liquid-like behavior.
The onset temperature, also referred to as the glassy dynamics’s melting temperature, acts as a crucial threshold. It effectively “melts” the supercooled liquid into a regular liquid state. This discovery has broad implications for all supercooled liquids and glassy systems, providing a fundamental understanding of their behavior.
Insights from Simulations
The researchers’ theory and simulations also capture other important characteristics of glassy dynamics. For instance, they found that while the majority of the liquid remains frozen, a small number of particles experience movement over short periods of time. This observation aligns with the state-of-the-art observations and adds to the microscopic understanding of the differences between supercooled liquids and regular liquids.
Future Directions
The team behind this breakthrough believes that their model can be expanded to three-dimensional systems. They also aim to delve deeper into how localized motions lead to nearby excitations, ultimately resulting in the relaxation of the entire liquid. By combining these components, a comprehensive microscopic picture of glassy dynamics can be developed, providing insights that bridge the gap between theory and experimental observations.
This newfound understanding of amorphous materials and their hidden phase transition has wide-ranging implications. It opens up possibilities for the development of novel amorphous materials that can be utilized in various fields such as medical devices, drug delivery systems, and additive manufacturing. By leveraging this knowledge, scientists can engineer materials with tailored properties to meet specific needs in these industries.
The discovery of a hidden phase transition in amorphous materials marks a significant advancement in our understanding of their behavior. By unraveling the microscopic mechanism behind the rigidity of supercooled liquids, researchers have laid the foundation for future developments in this field. With the potential to expand the model to three-dimensional systems and explore further details, scientists are well on their way to gaining a comprehensive understanding of glassy dynamics. This breakthrough has the potential to revolutionize various industries and pave the way for the design of advanced materials with targeted properties and functionality.

Leave a Reply